Oxidation Number of Magnesium
Oxidation continues rapidly as happens for a metal like magnesium Fig. If the ratio is equal to or slightly greater than unity an adherent non-porous protective oxide film forms such as typical on aluminium Fig.
Oxidation State Examples Online Chemistry Tutor
Thus the valency of nitrogen.

. Reduction is the gain of electrons or a decrease in the oxidation state of a chemical or atoms within it. CO oxidation on Rh is characterized by two well-defined stages depending on the catalyst temperature. Redox reductionoxidation ˈ r ɛ d ɒ k s RED-oks ˈ r iː d ɒ k s REE-doks is a type of chemical reaction in which the oxidation states of substrate change.
Daily Value DV 23 mg. Thus everything that leads back to magnesium metal in the. Difference between Valency and Oxidation Number.
Clinical and Experimental Im happy to share great news about the journal. Write the oxidation number of each atom in the skeleton equation. The reaction between magnesium oxide and carbon at 2000C to form magnesium metal and carbon monoxide is an example of the reduction of magnesium oxide to magnesium metal.
The high-voltage boron-based electrolytes also exhibit large potential in RMBs due to high oxidation stability high ionic conductivity and weak corrosion to current collectors. Preferential CO oxidation in hydrogen PROX on ceria-supported catalysts part I. However some of the elements exhibit very few oxidation States.
Citations are the number of other articles citing this article calculated by Crossref and updated daily. A mild and practical Csp3H lactonization protocol has been achieved by merging photocatalysis and magnesium iron nickel catalysis. Zns 2H aq Zn 2 aq H 2 g Another simple example is the reaction between copper oxide and magnesium to yield copper and magnesium oxide.
While fully ionic bonds are not found in nature many bonds exhibit strong. We report an oxygen-induced shape transformation of rhodium nanoparticles on magnesium oxide 001 substrates that is lifted upon. But the complex synthesis process and expensive raw.
Dear Friends and Colleagues As Editor-in-Chief of Metabolism. Oxidation state and surface species on PtCeO2 under reaction conditions. Therefore the valency of hydrogen is 1 as it can easily lose 1 electron and become stable.
Oxidation is the loss of electrons or an increase in the oxidation state of a chemical or atoms within it. Chop the cabbage into small pieces until you have about 2 cups of chopped cabbage. A diverse range of 2-alkylbenzoic acids with a variety of substitution patterns could be transformed into the corresponding phthalide products.
Valency is different from the oxidation number and it has NO SIGN. Place the cabbage in a. For example the oxidation of magnesium involves the chemical reaction between magnesium metal and oxygen to form magnesium oxide.
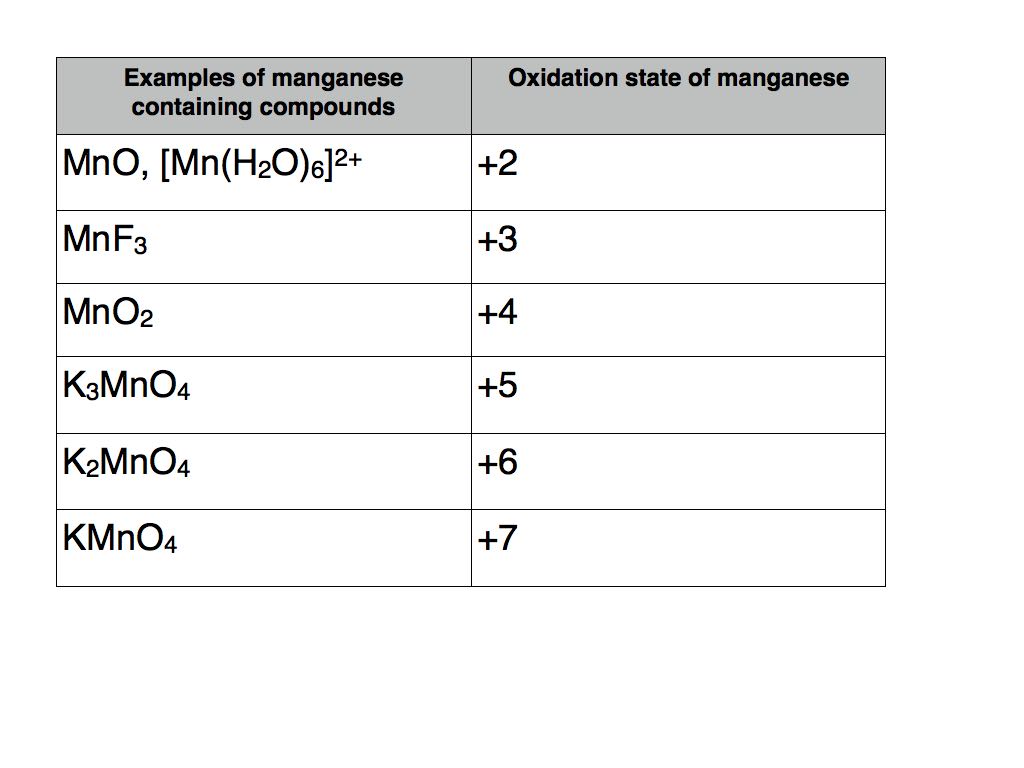
According to the number of magnesium papers published in 2021 the top 20 journals are listed in Table 1. For example magnesium shows a wide range of oxidation states that starts from 2 and goes up to 7 in its various compounds. In chemistry the oxidation state or oxidation number is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionicIt describes the degree of oxidation loss of electrons of an atom in a chemical compoundConceptually the oxidation state may be positive negative or zero.
The small number of oxidation states at the extreme left-hand side. On the other hand that of magnesium is 2 as it can lose 2 electrons easily and also attain stability. After electrons were discovered chemists became convinced that oxidation-reduction reactions involved the transfer of electrons from one atom to another.
Our Impact Factor has been continuously increasing over the past eleven years that I have been serving at the helm and is now at 13934 placing the journal amongst the top 4 of endocrinology diabetes and. Based on the mechanistic experimentation and reported prior studies a possible. The elements that are included in very few oxidation states are zinc and scandium.
The H ions with an oxidation number of 1 are reduced to H 2 with an oxidation number of 0 in the reaction. Antacids calcium carbonate calcium hydroxide magnesium hydroxide Seltzer water carbonic acid H 2 CO 3 Muriatic acid or masonrys cleaner hydrochloric acid HCl Lye potassium hydroxide KOH or sodium hydroxide NaOH Procedure. The word reduction comes from the to lead back sense of the Latin stem.

How To Find The Oxidation Number For Magnesium Mg Youtube

How To Find The Oxidation Number For S In Mgso4 Magnesium Sulfate Youtube

Belum ada Komentar untuk "Oxidation Number of Magnesium"
Posting Komentar